What is Pharmacogenomics?
Pharmacogenomics (or PGx) is the study of genetic variations that influence responses to healthcare medications. Pharmacogenomic testing enables doctors and healthcare professionals to test for specific genetic changes and predict whether a patient may have a normal or poor response, or a higher risk of side effects, before prescribing a specific medication.
myDNA provides a host of suitable reporting solutions and we use our Lab Gene by Gene for Lab services.
PGx is in our DNA

Associate Professor Les Sheffield
Clinical Geneticist & myDNA Co-Founder
Since the 1980s Professor Sheffield has been involved in the design and development of genetic tests. As a Clinical Geneticist, he has authored more than 100 scientific publications. In 2007 he founded myDNA to assist in more informed prescribing of medications. He continues to drive the development of new healthcare services as Medical Director.
Pharmacogenomics has long term healthcare benefits
Reduce the risk of adverse drug reactions
Reduce trial-and-error when prescribing
Offer a personalized healthcare service
Results have a lifetime relevance
Reduce wasted cost on ineffective medication
Fast-track treatment goals
What do we offer?
The myDNA Medication tests use PGx to identify variations in a number of genes that influence responses to medications and healthcare treatments.
We concentrate on the genes encoding the metabolizing enzymes (i.e. CYP450 enzymes) which are found mainly in the liver and the gut wall. These genetic variations predict the level of enzyme activity which in turn results in increased or decreased plasma concentrations and drug exposure.
Increased plasma concentrations, due to reduced enzyme activity, can lead to adverse effects. Reduced plasma concentrations, due to increased enzyme activity, can lead to poor therapeutic response.
We offer a multiple medications test, which covers multiple medication categories (currently in excess of over 80 medications). However, if a specific category is more suitable, we also offer single category medication reports.
Mental Health
Pain
Oncology
Gastrointestinal
Cardiovascular
PGx Healthcare in practice
Don’t waste time with trial-and-error
1 in 5 Australians
Suffer from persistent pain, making their risk of depression 4x higher than those without pain1.
50% of patients
Respond to the initial treatment with antidepressants2.
1 in 10 people
May process certain medications too slowly, increasing their risk of side effects3.
1 in 3 people
May process certain medications too quickly, increasing their risk of treatment failure3.
Patients most likely to benefit from our insights:
Those experiencing side effects to specific medications
Anyone not responding to specific medications
Patients requiring doses of specific medications outside the recommended range
Patients planning to start on a new medication covered by the myDNA test
Anyone taking multiple medications
- Painaustralia
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-17.
- Hicks JK, Sangkuhl K, Swen JJ, Ellingrod VL, Muller DJ, Shimoda K, et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther. 2017;102(1):37-44.
Mental health research conducted by myDNA
Mental healthcare inadequate for two thirds
Not everybody absorbs medications the same way. 50% of Australians have experienced a mental health issue in their lifetime, and nearly two-thirds believe their mental health treatment could be improved. myDNA can help alleviate the trial-and-error aspect of finding the right dose and ensure patients are taking both safe and effective doses.
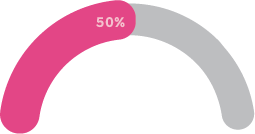
50% of Australians have experienced a mental health issue in their lifetime
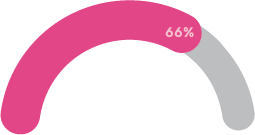
Two thirds believe their mental health treatment could be improved

One fifth believe they are not on the right drug or dose
How our medications test can change lives
Want to learn more?
Evidence behind pharmacogenomic testing
We reference the guidelines of the Royal Dutch Pharmacists Association, Pharmacogenetics Pharmacogenomics Working Group & the Clinical Pharmacogenetics Implementation Consortium (CPIC), which have been most recently published.
The following publications contain significant evidence for gene-drug associations of which dosage adjustments have been recommended.
- CPIC Guideline for CYP2D6 and CYP2C19 genotypes and dosing of Tricyclic Antidepressants
- CPIC Guideline for CYP2D6 and CYP2C19 genotypes and dosing of Selective Serotonin Reuptake Inhibitors (SSRIs)
- Dutch Pharmacogenetics Working Group guidelines which include a number of antidepressant and antipsychotic medications
Our scientific partner network
















A growing global network with the flexibility to scale and customize accordingly
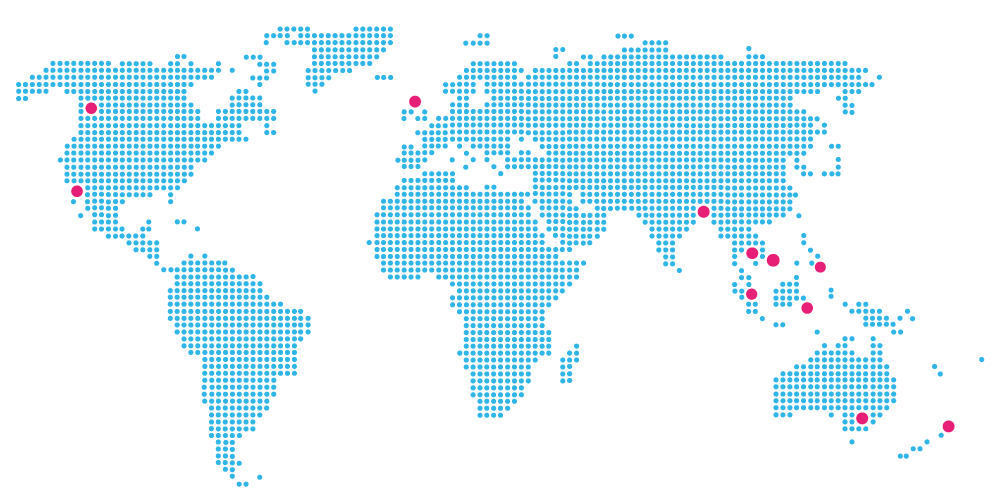
AUSTRALIA | NEW ZEALAND | USA | CANADA | UK | INDONESIA | SINGAPORE | THAILAND | PHILIPPINES | VIETNAM | BANGLADESH
Trusted accreditation on a global scale
Your data under lock and key
Your DNA sample and data remain your property, are stored on secure encrypted services, can be destroyed anytime at your request, and will never be shared without your consent.
Our analysis is limited to the DNA markers we report on. Your results won’t include diseases, can’t be used to identify you, nor will they have any bearing towards insurance policies.
Enquire about genomic reporting for your business
Leave your details below and our partnership team will be in touch.






